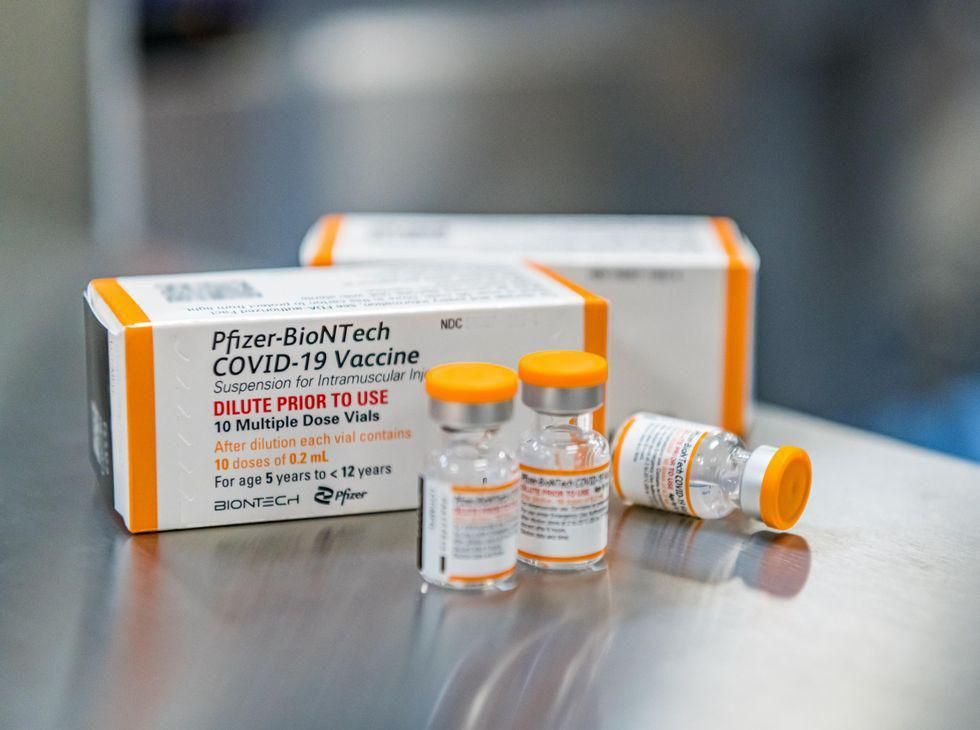
Pfizer Inc. said Wednesday that it has asked the U.S. Food and Drug Administration to approve the emergency use of its COVID-19 vaccine in children under the age of 5. The company said in a statement that it has provided the agency with data from a phase 2/3 trial that included almost 1,700 children who… read on > read on >